|
Supervised by: Dr Catherine Aiken (cema2@cam.ac.uk) & Professor Andrew Murray (ajm267@cam.ac.uk) |
|
|
Project Title |
Investigating placental energy metabolism in complex pregnancies using a novel experimental approach |
|
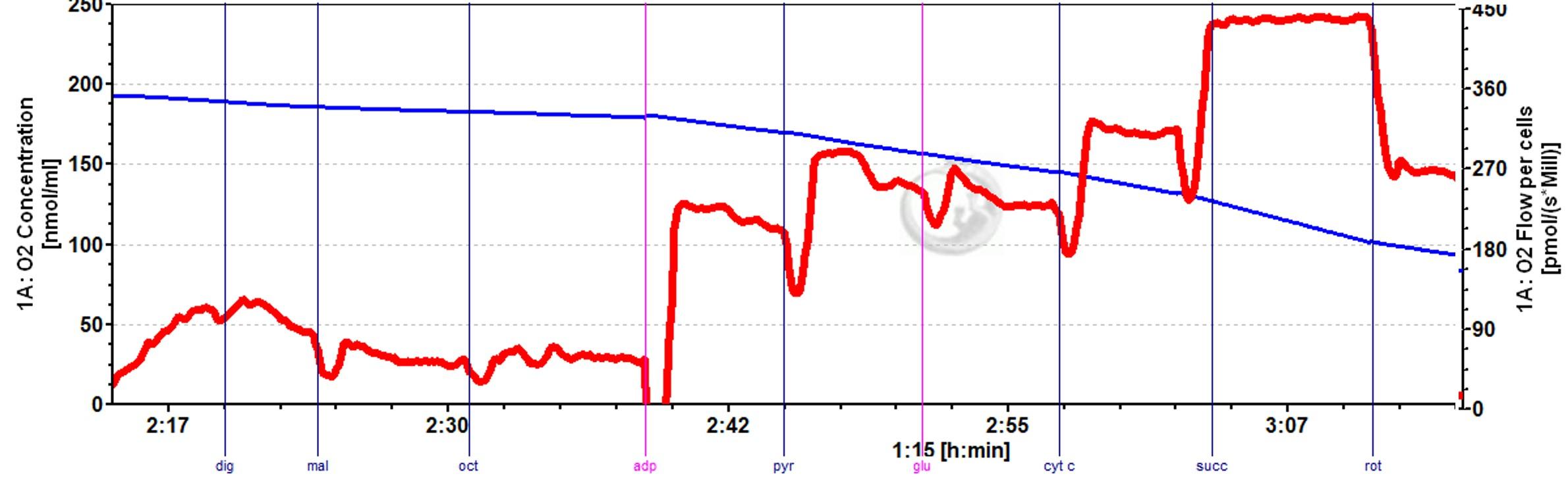
Project description As global populations face accelerating environmental challenges, increasing numbers of pregnancies are exposed to suboptimal conditions. Protecting populations from the impacts of these environments requires a close understanding of potential effects on the developing fetal-placental unit. This project focuses on utilising new experimental techniques, developed in our laboratories, to investigate the impact of various common environmental challenges on placental energy metabolism. Placental energy metabolism occurs at extremely high rates in late pregnancy in order to meet the demands of the growing fetus, while simultaneously maintaining the biosynthetic and signalling functions of the placenta itself. We have recently developed methods to assay the capacity for cellular respiration and to interrogate electron transport chain function in primary cells derived from human placentas. Our work makes use of high-resolution respirometry, a technique that can sensitively determine oxygen consumption in cells and tissues, thereby revealing detailed information about mitochondrial capacity, efficiency, and substrate utilisation. The key advantage to our experimental paradigm, using fresh cultured placental cells, is the robustness of the assay to understand common underlying variations in human populations e.g. maternal age. By creating a clean preparation of trophoblast from fresh placentas, and comparing experimental and control groups from each, we reduce the sample size required to detect differences and increase our confidence in drawing out true effects of each intervention. We can further explore the molecular impacts of interventions using a variety of other complementary techniques including gene expression studies, enzyme activity analysis and metabolomics as appropriate. The successful candidate will be expected to shape the precise focus of the project within the framework outlined, creating an excellent opportunity for a highly motivated individual to explore new areas or to bring their previous research interests and expertise to bear on this work. Examples of important and tractable specific aims include:
The techniques required for the project are established in both the Aiken and the Murray laboratories, thus maximising the time that can be spent addressing key scientific questions. There will also be opportunities and support to learn other molecular biology techniques necessary to further refine the hypotheses generated. Training in handling and culturing human tissue will be provided, in addition to career development skills such as scientific writing and data analysis. |
|
|
References
|
|

