|
Supervised by: Professor Dino Giussani (dag26@cam.ac.uk) and Professor Susan Ozanne (seo10@cam.ac.uk) |
|
|
Project Title: Obesity During Pregnancy: Adverse Effects on Maternal and Offspring Cardiovascular Function |
|
|
Host Department: Department of Physiology, Development and Neuroscience |
|
|
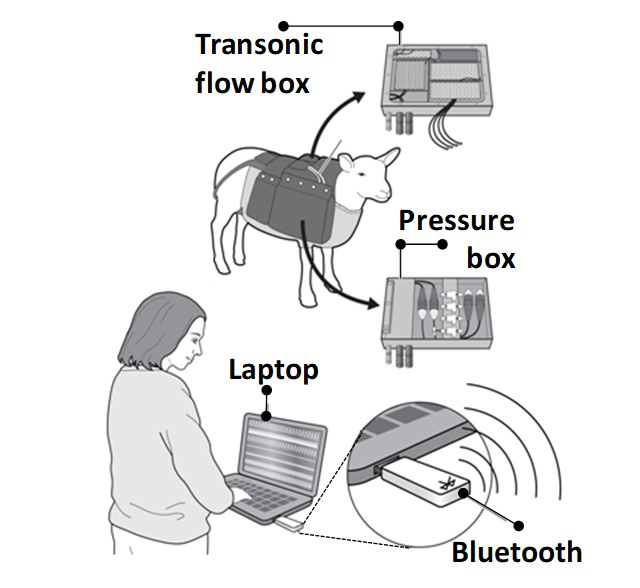
Project description Obesity has reached epidemic proportions, affecting more than 1.4 billion adults worldwide. The concern catapults to a much greater level of importance when considering maternal obesity. In her annual report, then as Chief Medical Officer, Dame Sally Davies highlighted that over half of women in the UK are now obese or overweight during pregnancy. This is of the gravest concern as obesity during pregnancy not only has immediate detrimental effects on the mother, but also on her children, thereby propagating adverse health risks onto the next generation. Rapidly accumulating evidence derived from human studies as well as preclinical animal models shows that maternal obesity can markedly increase the risk of cardiovascular disease in the offspring, even when the progeny feed on a healthy diet and in the absence of them becoming obese. This highlights that it is something about exposure of the embryo or fetus to an altered environment during obese gestation itself that either triggers a fetal origin of cardiovascular dysfunction and/or increases the risk of heart disease in the adult offspring. It is also known that complications during pregnancy can affect the maternal cardiovascular adaptations to pregnancy and her cardiovascular risk long after birth. However, the mechanisms linking obesity during pregnancy and an increased cardiovascular risk in the mother or offspring remain unclear, preventing targets for intervention. When studying pregnancy, the temporal profile of fetal development between species needs special consideration. In contrast to rats and mice models, sheep and humans share a close temporal profile of cardiovascular development and the maternal cardiometabolic adaptations to pregnancy including the maternal: fetal weight ratio is similar. Sheep and humans have similar uterine and umbilical blood flow, trans-placental oxygen gradients and nutrient transporter expression. Placental O2 consumption rates are nearly identical. After birth, access to suckling and the duration of weaning is also similar than humans. In addition, the sheep is the only established model that permits significant surgical instrumentation of the mother and fetus for long-term cardiovascular recording. Recently, we have made an important development in the Giussani Lab by designing the CamDASTM technology; a wireless data acquisition system that permits the simultaneous recording of multiple pressure and blood flow signals from mother and fetus in vivo over long periods of gestation in free-moving sheep. The proposed PhD project will exploit the novel CamDAS technology in our newly established ovine model of maternal obesity to test the hypothesis that maternal obesity during pregnancy triggers an increased cardiovascular risk in mother and offspring. References 3-5 below will give you a good idea of the type of work we do. |
|
|
References 1. Godfrey et al. (2017). Lancet Diabetes 5 (1): 53-64. |
|