|
Supervised by: Prof Francesco Colucci (fc287@medschl.cam.ac.uk) and Prof Dino A. Giussani (dag26@cam.ac.uk) |
|
|
Project Title: Immune gene variants and offspring health |
|
| Host Department: Department of Obstetrics & Gynaecology | |
|
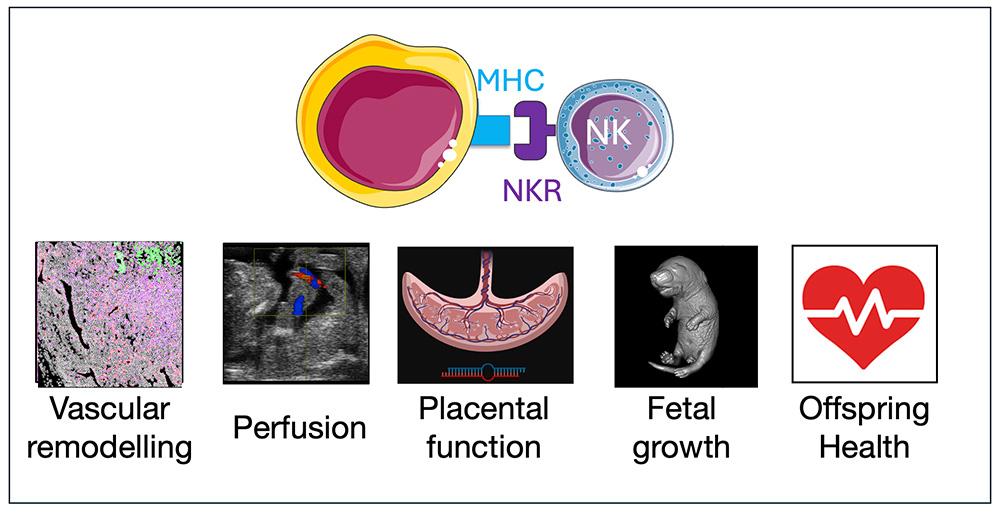
Project Description Genetic variants of major histocompatibility complex (MHC) and natural killer (NK) cell receptor (NKR) genes are linked with adverse pregnancy outcomes [1]. Nearly 20 million children are born underweight every year in the world; those with fetal growth restriction are exposed to greater risks of adult onset of cardiovascular and other diseases [2]. NK cells are abundant in the uterine microenvironment and, interacting with fetal trophoblast cells, they contribute to maternal adaptation to pregnancy and healthy fetal growth. Our hypothesis is that MHC and NKR variants known to lead to adverse maternal and fetal outcomes regulate the uterine micro-environment, and thereby cause disease onset in adult offspring. Our previous work has shown that a conserved NK-cell receptor (NKG2A) is responsible for optimal NK cell function in the uterus. Pregnant mice lacking the NKG2A receptor show utero-placental blood flow, reduced fetal growth and abnormal in-utero neurodevelopment [3]. New, unpublished data show postnatal catch-up growth and greater risk of adult onset of cardiac dysfunction in offspring from NKG2A KO dams. This shows that immune gene variants which regulate the uterine microenvironment programme cardiac dysfunction in mice adult offspring. Therefore, NK cells not only influence successful pregnancy, but they also have a role in modulating lifelong cardiovascular health in the offspring. However, the role of MHC and NKR gene variants in programming future risk of disease in the adult offspring is completely unknown. Our questions are: What are the in-utero mechanisms regulated by uterine NK cells that are central to healthy pregnancy and fetal growth? How do perturbations of these mechanisms lead to developmental origin of offspring cardiovascular disease? To determine how MHC and NKR gene variants regulate uterine mechanisms of healthy and pathological placentation, we will study the interactions between primary human NK cells and trophoblast organoids derived from human trophoblast stem cells that express relevant MHC and NKR genes. To determine the effects of perturbations of these interactions on offspring cardiovascular disease we will determine cardiac function under basal and stimulated conditions using well-established isolated Langendorff heart preparations, and indices of cardiac injury, and molecular analysis of key pathways [4,5]. Current therapeutic options for pregnant women who have experienced adverse pregnancy outcome are limited. Mechanistic understanding of how maternal immune cell function determines placental development with consequences on fetal and offspring health could lead to novel immunotherapies to prevent both perinatal complications and long-term disease in the offspring. |
|
|
References
|
|
|
Candidate background requirements The candidate ought to ideally have completed an MPhil working on a project related to either lymphocytes or cardiovascular biology |
|

